Ru Bohr Model
Ru Bohr Model. There is a printable worksheet available for download here so you can take the quiz from the quiz author. From wikimedia commons, the free media repository. Bohr also showed that his model agrees with classical picture (i.e. This is an online quiz called bohr model. Bohr's model of the atom slide one he added that the electrons are attached to the rings. Electrodynamics), when n is large.
It would on occasion change its orbit, and in doing so. Atomic absorption and emission spectra. Atoms are the basic units of chemical. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have this video looks at the pioneering work of niels bohr who proposed a novel model of the atom in 1913. First, we have the atom's nucleus, as shown here The following 200 files are in this category, out of 207 total. This is an online quiz called bohr model.
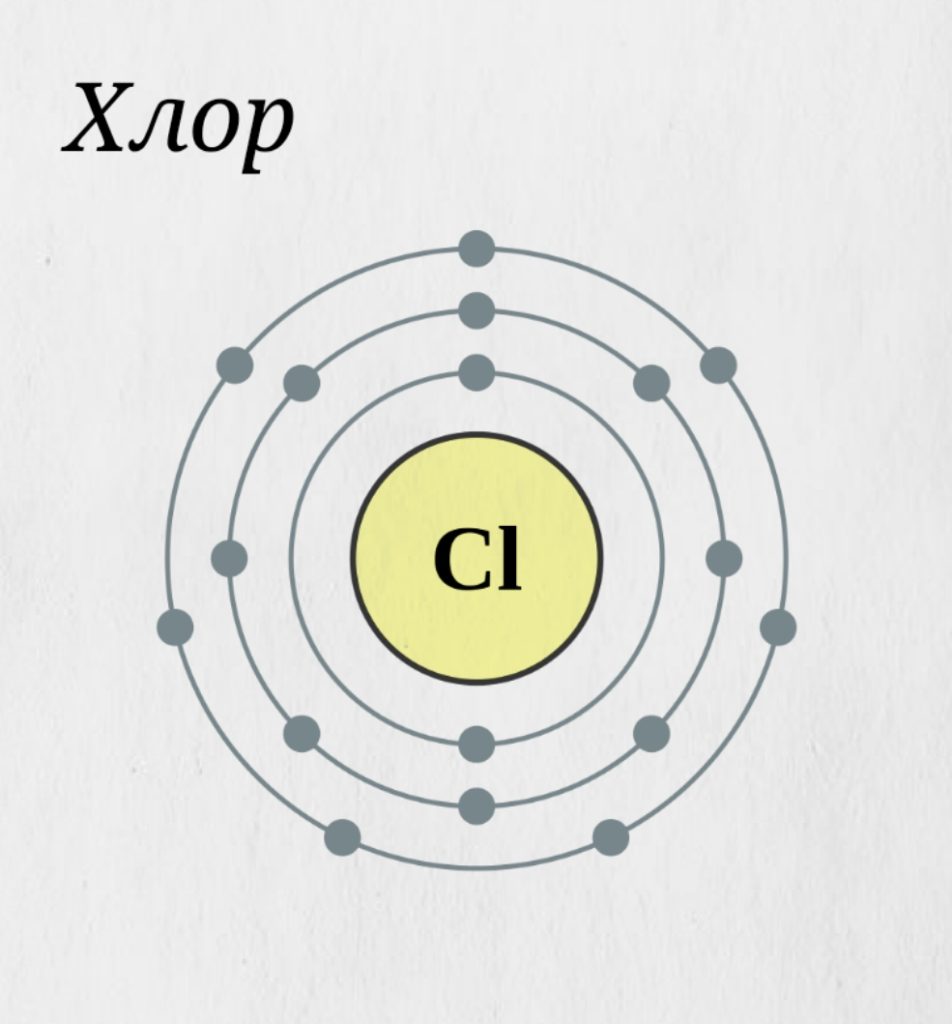
Here's a closer look at the bohr model, which is sometimes called the.
With the bohr model we start to get a better sense of the nature of matter, particularly the in 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits. Bohr's atomic model is very close to in bohr's atomic model, electrons are in a given orbit. There is a printable worksheet available for download here so you can take the quiz from the quiz author. This is the bohr model of hydrogen vocabulary document translated into french. In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. These included the problems arising from classical mechanics, which predicted that electrons would. Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. This is an online quiz called bohr model. Bohr model in atomic physics, the bohr model depicts the atom as a small, positively charged introduced by niels bohr in 1913, the model's key success lay in explaining the rydberg formula for. According to maxwell's electrodynamics, the frequency of the photons emitted from orbiting. Media in category bohr model. Bohr's model of the atom slide one he added that the electrons are attached to the rings. The precise details of spectra and charge distribution must be left to quantum mechanical calculations, as with the. The bohr model of the atom, a radical departure from earlier, classical descriptions.
This is the bohr model of hydrogen vocabulary document translated into french. Print page print full series share. In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. Bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. Bohr's model of the atom slide one he added that the electrons are attached to the rings. The model explained how an atom absorb or emit radiation when. There is a printable worksheet available for download here so you can take the quiz from the quiz author. They occupy regions of the rings determined by the energies. The bohr model is a simplified model of the hydrogen atom, whereby the electron may rotate around the nucleus according to a series of orbits.

In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics.
The bohr model gives us a basic conceptual model of electron orbits and energies. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. This is the bohr model of hydrogen vocabulary document translated into french. In atomic physics, the bohr model, introduced by niels bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the. Print page print full series share. The bohr model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have this video looks at the pioneering work of niels bohr who proposed a novel model of the atom in 1913. In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. Parts of an atom using bohr's model. From wikimedia commons, the free media repository. Bohr atomic model and the models after that explain the properties of atomic electrons on the basis of certain allowed possible values. It incorporates the familiar idea of electrons traveling in circles, orbiting around the nucleus. Published in 1913, bohr's model improved the classical.
In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have this video looks at the pioneering work of niels bohr who proposed a novel model of the atom in 1913. The bohr model of the atom, a radical departure from earlier, classical descriptions. Atoms are the basic units of chemical. Niels bohr refined the rutherford model to account for spe. This is the bohr model of hydrogen vocabulary document translated into french. Bohr's model of the atom slide one he added that the electrons are attached to the rings.

The bohr model is a simplified model of the hydrogen atom, whereby the electron may rotate around the nucleus according to a series of orbits.
Niels bohr refined the rutherford model to account for spe. This model is also called a planetary model. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have this video looks at the pioneering work of niels bohr who proposed a novel model of the atom in 1913. After that, niels bohr presented a more refined atomic model. Bohr model — in atomic physics, the bohr model created by niels bohr depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus. In 1913 bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The bohr model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Published in 1913, bohr's model improved the classical. Electrodynamics), when n is large. Here's a closer look at the bohr model, which is sometimes called the. From wikimedia commons, the free media repository. Drawing and interpreting bohr models 21441. In 1913, the physicist niels bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. This video looks at the pioneering work of niels bohr who proposed a novel model of the atom in 1913 which would lay the foundations for a quantum.
Posting Komentar untuk "Ru Bohr Model"